Introduction to Ultrasounds
Being a sound wave, ultrasound is transmitted through any substance, solid, liquid or gas possessing elastic properties. The movement of a vibrating body (the sound source) is communicated to the molecules of the medium, each of which transmits the motion to an adjoining molecule before returning to approximately its original position.
In liquids and gases, particle oscillation takes place in the direction of the wave and produces longitudinal waves. Because they additionally possess shear elasticity, solids can also withstand tangential stress and produce transverse waves, where particles move normal to the direction of the wave. One example of a transverse wave is that obtained when a stone is dropped into a pool of water. On the other hand, a longitudinal wave is produced, for example, when a coiled spring anchored at one end is given a sharp push from the other end.
The action causes a disturbance in the spring which “runs” through the whole length by expansion and compression.
If the disturbance in the pool or spring is periodically repeated, expansion and compression cycles traveling through a medium will occur. Compression cycles push molecules together, whereas expansion cycles pull them apart. In a liquid, the expansion cycle produces negative pressure that pulls molecules away from one another. If the Ultrasound intensity is high enough, the expansion cycle can create bubbles or cavities in the liquid. Such is the case when the negative pressure exerted exceeds the local tensile strength of the liquid, which varies depending on its nature and purity.
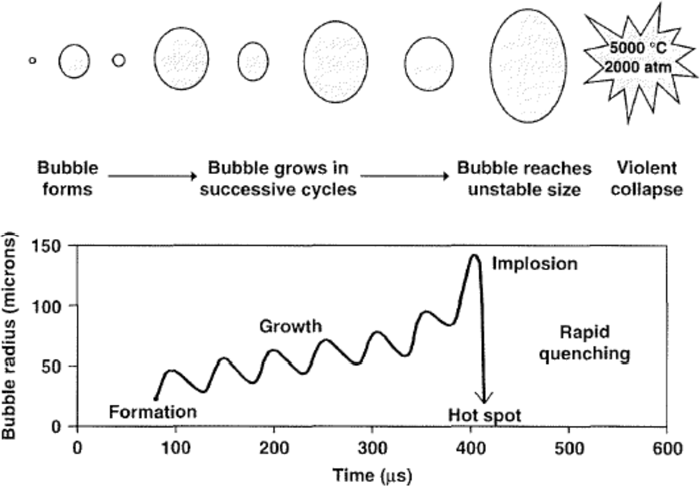
The process by which bubbles form, grow and undergo implosive collapse is known as “cavitation”. The steps involved in the process are depicted in Fig. 1.
1.1. Applied Frequency
The significance of cavitation to sonochemistry is not so much on how the bubbles form, but rather what happens when they collapse. At some point, a bubble can no longer absorb the energy efficiently from the ultrasound so it implodes. Rapid adiabatic compression of gases and vapors within the bubbles or cavities produces extremely high temperatures and pressures. Suslick estimated the temperature of these hotspots to be about 5000°C, which is equivalent to that on the surface of the sun. The pressure is roughly 2000 atm, which is higher than that at the Marianas Trench ‐ the deepest point in the ocean.
The size of the bubbles is very small relative to the total liquid volume, so the heat they produce is rapidly dissipated with no appreciable change in the environmental conditions‐ this is why cavitation is also known as “cold boiling”. The cooling following the collapse of a cavitational bubble has been estimated to be in the region of 10 billion ºC/s ‐ a million times faster than that of a red‐hot iron rod plunged into the water. Acoustic cavitation thus provides a unique interaction between energy and matter, which has been characterized by using electrochemical, luminescent and photographic techniques.
When cavitation occurs in a liquid close to a solid surface, the dynamics of cavity collapse change dramatically. In pure liquids, the cavity retains its spherical shape during collapse as its surroundings are uniform. Close to a solid boundary, however, cavity collapse is rather asymmetric and produces high‐speed jets of liquid. Liquid jets driving into the surface at speeds close to 400 km/h have been observed. The impact of the jets on the solid surface is very strong. This can result in serious damage to impact zones and produce newly exposed, highly reactive surfaces. Distortions of bubble collapse depend on the surface several times larger than the resonant size of the bubble. This is the reason why sonotrodes are made of harder materials like Titanium.
1.2. Factors influencing cavitation
This section examines variables influencing the cavitation phenomenon in a manner that can be adjusted to fulfill specific purposes.
1.2.1. Gas and particulate matter
The acoustic pressure needed to cause cavitation in water has been estimated to be approximately 1500 atm. In practice, cavitation occurs at considerably lower values (<20 atm) as a result of the presence of weak spots in the liquid that reduce its tensile strength. There is now sufficient experimental evidence to suggest that one cause of weak spots is the presence of gas molecules in the liquid. Thus, degassing a liquid has been found to raise the cavitation threshold (i.e. the applied acoustic pressure required for cavitation bubbles to form). Increasing the gas content of a liquid lowers the cavitational threshold and reduces the intensity of the shock wave released as the bubble collapses. Thus, when the primary source of sonochemical effects is a cavitational collapse, a bubbled gas should be used to produce a large number of nucleation sites. Monoatomic gases are preferable to diatomic and polyatomic gases for this purpose. Thus, He, Ar and Ne are used in preference to diatomics such as N2, air or O2; on the other hand, gases such as CO2 are the most unsuitable.
The presence of particulate matter‐ especially that of trapped vapor gas nuclei in their crevices and recesses‐ has also been found to lower the cavitation threshold. This is required under these conditions are of paramount importance in the clinical field, where ultrasound is widely used in applications such as high‐ intensity focused ultrasound (HIFU), extracorporeal shock wave lithotripsy (ESWL) and sonodynamic therapy.
Also, research on the bubble and bubble cloud dynamics in the clinical range of ultrasound frequency and intensity is an ongoing task.
1.2.2. External (applied) pressure
Increasing the external pressure raises the rarefaction pressure required to initiate cavitation. The application of external pressures which would cause any suspended gas molecules to dissolve, thereby effectively removing the gas nuclei, has also been found to raise the cavitation threshold. More importantly, increasing the external pressure increases the cavitation collapse intensity and as a result, enhances sonochemical effects. Qualitatively, there should no longer be a resultant negative pressure phase of the sound wave, so no cavitation bubbles should form.
1.2.3. Solvent viscosity
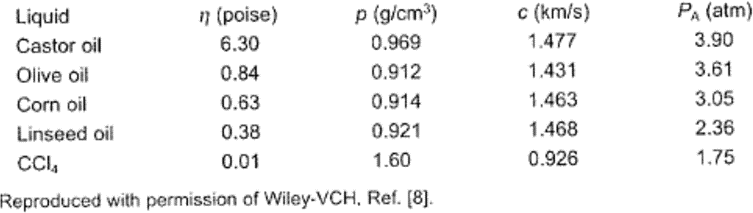
Because the negative pressure in the expansion or rarefaction cycle must overcome the natural cohesive forces acting in the liquid, any increase in such forces will raise the cavitation threshold. One way of increasing these forces is by increasing the viscosity of the liquid. Table 1 illustrates the influence of viscosity on the pressure amplitude (PA at which cavitation starts in various liquids at 25 ºC, at a hydrostatic pressure of 1 atm. The effect, though not insignificant, is hardly dramatic.
1.2.4. Solvent surface tension
Cavitation requires the formation of a liquid‐gas interface. Thus, one might expect the use of a solvent of low surface energy per unit area to lower the cavitation threshold. Although the phenomenon is not as simple as it may seem, the addition of a surfactant to an aqueous solution certainly facilitates cavitation.
1.2.5. Solvent vapor pressure
Cavitation bubbles do not enclose a void. During the expansion phase of cavitation bubble generation, vapor from the surrounding liquid will permeate the interface. This produces a small pressure within the bubble, thereby reducing the pressure difference between cavity and bulk. It is difficult to induce cavitation in a solvent of low vapor pressure because less vapor will enter the bubble. A more volatile solvent will support cavitation at lower acoustic energy and produce vapor-filled bubbles.
Unfortunately, sonochemical effects are based on the energy produced by cavitation bubble collapse, which is cushioned by vapour in the bubbles. Hence, solvents with high vapour pressures easily generate vapour‐filled bubbles, but their collapse is cushioned and therefore less energetic.
1.2.6. Applied frequency
As the frequency of irradiation is increased, the rarefaction phase shortens and the amplitude (power) of irradiation has to be increased to maintain an equivalent amount of cavitational energy in the system. In other words, more power is required at a higher frequency if the same cavitational effects are to be maintained. This is due to the fact that completely rupturing a liquid to obtain voids that may be subsequently filled with gas or vapor requires a finite time.
With high‐frequency sound waves, the time required to create a bubble may exceed that available during the rarefaction cycle. At 20 kHz, for example, the rarefaction cycle lasts 25 µS and reaches its peak negative pressure in 12.5 µS; at 20 MHz, however, the rarefaction cycle lasts only 0.025 µS. One might thus anticipate that, as the frequency increases, producing cavitation bubbles during the available time will be increasingly difficult and increased sound intensities (i.e. greater amplitudes) will have to be employed over the shorter periods to ensure that cohesive forces in the liquid are efficiently overcome.
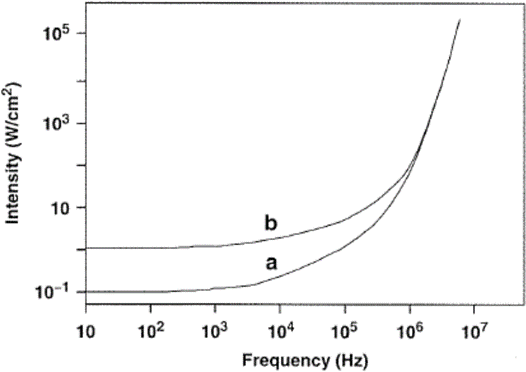
This effect is illustrated in Fig. 2, which shows the variation of the threshold intensity with the frequency for both aerated and gas‐free water. As expected, the threshold for aerated water is lower than that for gas‐free water, and the threshold intensity increases with increase in frequency. In fact, ten times more power is required to make water cavitate at 400 kHz than at 10 kHz. For this reason, frequencies in the range 20‐50 kHz have traditionally been used for cleaning purposes and subsequently been found useful in sonochemistry.
As can be seen in Fig. 2, little additional energy is required to cause water to cavitate at 50 kHz relative to 1 0 kHz. Below 18 kHz, however, there may be some noise discomfort to the user since this frequency is within the audible range. This is why sonochemical applications normally involve frequencies above 20 kHz.
1.2.7. Temperature
In general, the cavitation threshold has been found to increase with decreasing temperature.
This may in part be due to an increase in either the surface tension or viscosity of the liquid as the temperature decreases or to a decrease in the liquid vapor pressure. Increasing the temperature facilitates cavitation at a lower acoustic intensity. This is a direct consequence of the rise in vapor pressure associated with heating the liquid – at higher temperatures near the solvent boiling point, a large number of cavitation bubbles are generated concurrently. The higher is the vapor pressure the lower will be the applied acoustic amplitude required to ensure that the “apparent” hydrostatic pressure is exceeded. Unfortunately, the effects of cavitational collapse are also reduced as the bubbles will act as a barrier to sound transmission and dampen the effective ultrasonic energy from the source entering the liquid medium. Therefore, in order to maximize sonochemical effects, any experiment should be conducted at as low a temperature as possible or by using a solvent of low vapor pressure. The mathematical equations that relate these variables and their effects on the bubbles are beyond the scope of this article and can be found elsewhere.
1.2.8. Intensity
The sonication intensity is directly proportional to the square of the vibration amplitude of the ultrasonic source. As a rule, increasing the intensity increases the sonochemical effects; however, the ultrasonic energy a system can take is limited. Thus, cavitation bubbles, which are initially difficult to create at the higher frequencies as a result of the shorter duration of rarefaction cycles, are now possible by virtue of the collapse time, temperature and pressure on collapse being mutually dependent. However, the sonication intensity cannot be increased indefinitely as the maximum possible bubble size is also dependent on the pressure amplitude. As the pressure amplitude is increased, bubbles may grow so large on rarefaction that the time available for collapse will be inadequate.
In fact, it has been unequivocally established that:
- A minimum sonication intensity level is required to reach the cavitation threshold.
- When a large amount of ultrasonic power enters a system, the solution produces a large number of cavitation bubbles many of which coalesce into even larger, longer‐lived bubbles that will hinder the transport of acoustic energy through the liquid.
- At high vibrational amplitudes, the Ultrasonic source will be unable to maintain contact with the liquid throughout the cycle. This is technically known as decoupling and considerably reduces the efficiency with which power can be transferred from the source to the medium. Decoupling is especially strong when large numbers of cavitation bubbles build up at or near the emitting surface of the transducer.
- The transducer material used in the sonicator will eventually break down as the size changes in the transducer grow large enough to fracture the material.
1.2.9. Field Type
Acoustic cavitation is empirically known to be induced much more efficiently and reproducibly in a standing wave field than in a progressive one; also suspended particles exposed to an ultrasound standing wave are known to be driven by an axial force to concentrate in nodal or antinodal planes. This phenomenon has received substantial attention in recent years. The magnitude of Rayleigh microstreaming convective drag on microparticles in water in a 3.2 MHz ultrasonic standing wave has been shown to be comparable to the lateral direct radiation force in the nodal plane and to significantly influence microparticle aggregation.
Using single half-wavelength chamber the transducer was excited in order to obtain a single particle aggregate with an estimated sound pressure amplitude of 0.5 MPa; the microstreaming velocity in the nodal plane was calculated from the particle image velocity and found to be comparable to the value obtained in the light of Rayleigh’s theory. Further evidence of the significance of microstreaming was provided by the fact that the velocities for particles 1 and 25 µm in size were similar in magnitude, but opposite in direction.
Recently, this phenomenon was found to be induced at a relatively low ultrasonic intensity, even with progressive waves by the second harmonic overlapping the fundamental one. This finding is of paramount importance as regards the clinical applicability of US, and also in sonochemical uses involving systems sensitive to high ultrasound intensities.
1.2.10. Attenuation
For a variety of reasons, the intensity of US is attenuated (i.e. decreased) as it progresses through the medium. Part of the energy is dissipated in the form of heat, which is hardly appreciable in the bulk medium during sonication. The extent of attenuation is inversely proportional to the sonication frequency. This can be illustrated in the case of sound attenuation through pure water. Thus, the US at 118 kHz is reduced to half its original intensity after passing through 1 km of water.
At 20 kHz, the distance required to achieve the same reduction in intensity is much greater (30 km). Therefore, obtaining identical intensities in a medium at a given distance from an ultrasonic source at variable frequencies entails using a higher initial power in the sources with the higher sound frequencies.
1.2.11. Types of ultrasound cavitation
Depending on the particular type of bubbles, ultrasound cavitation can be transient or stable. In the transient type, also known as inertial cavitation, bubbles are either voids or vapor bubbles, which are believed to be produced by intensities above 10 W/cm2. They exist for one, or at most a few acoustic cycles, and expand to a radius of at least twice their initial size before collapsing abruptly on compression and often disintegrating into small bubbles. The smaller bubbles formed can act as nuclei for further bubbles or, if their radius is sufficiently small, they can simply dissolve into the bulk solution under the action of the very large surface tension forces present. The lifetime of transient bubbles is believed to be too short to allow any mass flow by diffusion of gas into or out of the bubbles; by contrast, evaporation and condensation of liquid are believed to occur freely. In the absence of gas to cushion the implosion, the bubbles will collapse highly abruptly.
Theoretical considerations by Noltingk and Neppiras, subsequently expanded by Flynn and Neppiras, that assume the adiabatic collapse of the bubbles, allow the temperature and pressure within the bubble at the time of total collapse to be calculated. Stable or non‐inertial cavitation was at one time thought to be of little significance in terms of chemical effects. Thus, stable bubbles are believed to contain mainly gas and some vapor, and to be produced at fairly low intensities (1‐3 W/cm2); also, they are assumed to oscillate, often in a non‐linear manner, about some equilibrium size over many acoustic cycles. Their timescale is sufficiently long for mass diffusion of gas and thermal diffusion to occur, and hence for evaporation and condensation of vapor, which is bound to have significant long‐term effects. Differences in mass transfer rate across the gas‐liquid interface may result in bubble growth. The mechanism by which small microbubbles in the liquid ‐ which normally dissolve instantly under surface tension ‐ grow is termed as “rectified diffusion”.
In the expansion phase of the acoustic cycle, gas diffuses from the liquid into the bubble; in the compression phase, gas diffuses out of the bubble into the liquid. Because the interfacial area is greater in the expanded phase, inward diffusion is greater than outward diffusion and results in the overall growth of the bubble. As the bubble grows, the acoustic and environmental conditions of the medium will change and the bubble may become a transient bubble and collapse ‐ less abruptly than a vapor-filled transient bubble, however, as the implosion will be cushioned by the gas. Tests involving new, more sophisticated measurement tools have provided new interpretations and equations for the cavitation phenomenon. The thermal and non‐thermal effects of non‐inertial cavitation and the chemical and mechanical effects of inertial cavitation in relation to their impact on ultrasound safety have recently been investigated.
Cavitation is not exclusive of ultrasound. Thus, hydrodynamic cavitation can simply arise from passage of the liquid through a constrictor such as a throttling valve, orifice plate, Venturi tube, etc. On passage through the constrictor, the kinetic energy‐ velocity of the liquid increases at the expense of the pressure. Various types of hydrodynamic cavitation reactors have been reported and their most salient features reviewed. Also, the effects of ultrasound and hydrodynamic cavitation on oxidation processes have recently been compared.
1.3. Chemical Aspects of Ultrasound
The high temperature and pressure created within a collapsing cavitation bubble produced by Ultrasound radiation cause the formation of free radicals and various other species; thus, sonication of pure water causes its thermal dissociation into H and OH radicals, the latter forming hydrogen peroxide by recombination. These radicals constitute one of the essential pieces of evidence for the phenomena classified as sonochemistry.
Table 2 shows the main reactions occurring in water irradiated with Ultrasound. Some of the radical species generated, which have been detected in a number of ways including spin trapping, have been cited as potential pollutant destruction agents on the grounds of their extremely high redox potentials (e.g. P =+ 2.8 V for OH radicals. There is less evidence for the behavior and detection of H, even though, in principle, it is produced in amounts equivalent to those of OH∙ in the primary solvent degradation step (see Table 2).
The two radicals are rather different in chemical nature, however. Thus, the OH radical is known to initiate a number of reactions in solution; by contrast, the H radical can be rapidly captured by molecular oxygen. If the water contains some salt such as potassium iodide or copper sulfate, then other free radicals in addition to the species in Table 2 can be expected to be released. Another major chemical phenomenon related to ultrasonic cavitation is sonoluminescence, by which a tiny light is formed in a cool liquid. This form of light emission results from the high‐ temperature formation of reactive chemical species in excited electronic states. Emitted light from such states provides a spectroscopic probe for the cavitation effect. Some electrical and thermal theories on this phenomenon have been reported.
1.3.1. Some rules of sonochemistry
Based on the existing sonochemical literature, ultrasonic irradiation seems to have been developed on a practical rather than on a theoretical basis. The justification for enhanced reactivity and process acceleration, in general, were largely rationalized by using the intuitively straightforward “hot spot” approach. Critics of ultrasound regarded it merely as a super‐ agitation tool. Careful examination and classification of published material on sonochemistry have allowed an empirical classification to be established. While the classification focuses on the chemical effects of sonochemistry, it should also be recognized that, in some cases, Ultrasound does act in a mechanical sense and provides outstanding results through super agitation. Occasionally, the mechanical and chemical effects occur simultaneously. The three rules derived from published material on sonochemistry are as follows:
Rule 1. Applies to homogeneous processes and states that the reactions which are sensitive to the sonochemical effect are those that proceed via radical or radical‐ion intermediates. This means that sonication can affect reactions proceeding through radicals and that ionic reactions are not likely to be modified by such irradiation.
Rule 2. Applies to heterogeneous systems, which are more complex and where reactions proceeding via ionic intermediates can be stimulated by the mechanical effects of cavitational agitation. This has been termed “false sonochemistry”, even though many industrialists may argue that the word “false” is incorrect here because if ultrasonic irradiation assists a reaction, the reaction is indeed assisted by sonication and thus “sonochemical”. In fact, the true test for “false sonochemistry” is that similar results should, in principle, be obtained by using an efficient mixing system in place of sonication. Such a comparison is not always possible, however.
Rule 3. Applies to heterogeneous reactions with mixed mechanisms (i.e. radical and ionic). These will have their radical component enhanced by sonication, even though the general mechanical effect from Rule 2 may still apply. There are two possible situations for heterogeneous systems involving two different mechanism paths. If the two mechanisms lead to the same product(s) (i.e. the process is “convergent”), the sole effect will be an increase in the overall rate. On the other hand, if the radical and ionic mechanisms lead to different products, then sonochemical switching will be possible through a favored radical pathway. In such “divergent” processes, sonication actually changes the nature of the reaction products. Note that a very high‐speed stirrer can have effects similar to those of sonication on heterogeneous systems. This may well be a case with the processes induced by hydrodynamic cavitation rather than acoustic cavitation. The physical effects of ultrasound in relation to analytical chemistry have not yet been rationalized.
2. The Application of Ultrasounds in the Shipping and Industry
Enermulsion is a patented ultrasonic cavitation technology, that decomposes the water molecules by thermionic and crakes the chain of hydrocarbons, the obtained radicals become attached to the hydrocarbon cracked molecules which improve the fuel oil combustion properties. This technology allows the reduction of exhaust gases emissions (such as NOx) and at the same time saves fuel and a cleaner engine.




1 comment on “Introduction to Ultrasounds”
That’s pretty cool that it’s possible to make an image with sound. It’s even cooler that it make sure possible to see through things and see things like a baby. I wouldn’t have guessed that sound was capable of doing that.